Barium Chromate is the oxidizing agent and the state of the Chromium oxidation in this compound is +6. The Barium ions in the compound generate green flames when heated. When the question is to know the solubility of barium chromate is parts per million 3.652 * 10^-7 ppm then, we are here with the exact answers for the same.
Let’s move ahead with the exact answers for the same: what are the methods of solubility of barium chromate in parts per million.
Solubility of a provided compound is referred to as a certain temperature as well as dissolvable. It suggests that you will need a little information or details or beginning data. For this scenario, you can use the molar solvency of the solver chromate in the water at 25.0 °C. The molar solvency of the silver chromate is 1.10 * 10 ^ – 12 M.
You may also like: How to discover Music and Pop Culture in 2021?
Given that the concentration of the arrangement is too low its thickness is almost nearly similar to water thickness of water, for instance, 1g/Ml. At that point, you have 1.10 – 10 ^ – 12 mol of silver chromate/1000g of arrangement. Now, convert the moles of silver chromate in grams, using the molar mass.
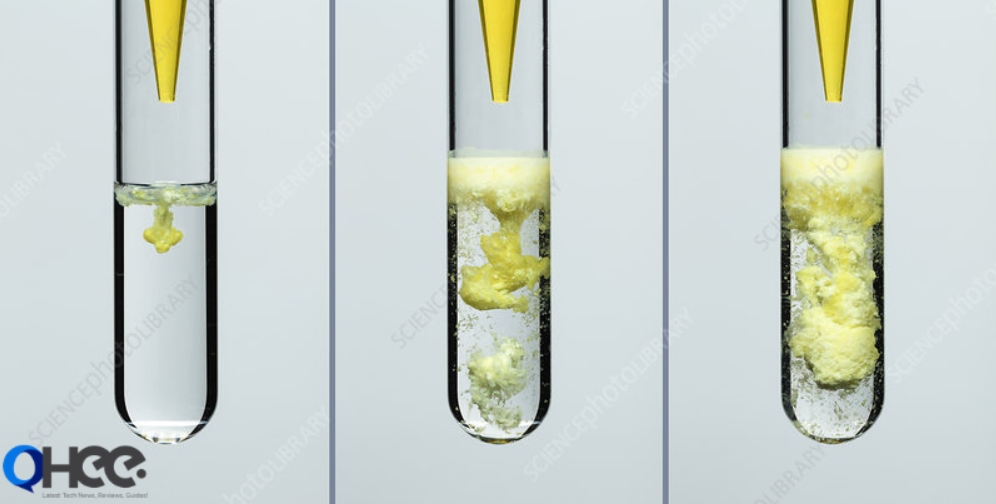
Silver Chromate is Ag2CrO4 and Molar mass of Ag2 CrO4 = 2*108g/mol + 52g/mol + 4*16g/mol = 332 g/mol 1.10 * 10 ^ – 12 mol* 332 g/mol = 3.652 * 10^ – 10g.
That is the grams quantity in 1000 gms of arrangement, at that point, you have to increase times 1000 to make changes 1,000,000 grams of arrangement = 3.652 10^-10 *10^3 grams of Ag2Cr04/1,000,000 grams of arrangement = 3.652 *10^-7 grams of Ag2Cr04/1,000,000 grams of arrangement. You have to keep in mind ppm is parts/million, at that point, it is 3.652*10^-7 ppm.
Silver Chromate
Silver chromate is a dark-colored red monoclinic gem and it is a substance forerunner to recent photography. It seems to be framed by combining silver nitrate and sodium chromate of potassium chromate.
Read More: What is the Avast URL blacklist and How to Manage it?
Production of Barium Chromate
Barium Chromate is generated by the precipitation reaction of Barium Chloride (BaCl2) and Sodium Chromate (Na2CrO4) and the reaction happens in the given following way that is:
BaCl2 + Na2CrO4 → BaCrO4 ↓ +2 NaCl
This reaction generates BaCrO4 precipitation that is after that gathered by filtration and Sodium Chloride is also generated NaC1 in this reaction. The molecular formula for this compound is BaCr04.
Barium Chromate Usages: (Extensively used for many industrial purposes)
It is extensively used as an oxidizer and burn rate modifier as well in many pyrotechnic compositions (basically a substance that generates an effect by sound, light, heat, smoke, and gas as a self-sustaining exothermic reaction result) specifically delay compositions.
- This compound is widely used to prevent corrosion between dissimilar metals
- It is extensively used as a pigment in paints, fuses, porcelains, and coloring glasses
- Barium Chromate is extensively used in safety matches and is also useful in various ignition control devices
This compound is widely used in metal primers and the substance is widely used in explosives as an explosions initiator.